barostim neo system
The BAROSTIM NEO Hypertension Trial is a prospective randomized controlled trial assessing the safety and efficacy of the BAROSTIM NEO System in subjects with resistant hypertension. These receptors in the neck sense the blood that is flowing through the carotid arteries.
Cvrx S Barostim Neo Gets Ce Mark For Use With Mris Massdevice
The neo non-randomized hypertension study is a non-randomized open-label verification study in participants diagnosed with drug resistant hypertension defined as medical treatment failure for hypertension defined as office cuff systolic blood pressure sbp 140 mmhg despite being prescribed to at least three antihypertensive medications.

. Barostim neo system is indicated for the improvement of symptoms of heart failure quality of life six-minute hall walk and functional status for patients who remain symptomatic despite treatment with guideline-directed medical therapy are nyha class iii or class ii who had a recent history of class iii have a left ventricular ejection. Barostim Neo is a neuromodulation system developed by CVRx for the treatment of heart failure and hypertension. In the model Barostim reduced over a lifetime the rates of myocardial infarction by 19 stroke by 35 heart failure by 12 and end-stage renal disease by 23.
This pacemaker-like device is designed to electrically activate the baroreflex the bodys main cardiovascular reflex which signals the brain to regulate heart function. Office Cuff Blood Pressure. Learn More Patient stories Im able to do the things Ive always wanted to do -David Im back to walking three to four miles a day -Kevie Amazingly happy tears of joy I havent had in 10 years -Rhonda.
The FDA approval for the CVRx Barostim Neo System is based on the data from a multi-center two-arm randomized clinical trial the BeAT-HF phase 3 study featuring 408 patients with advanced heart failure. Res The brain works to counteract this perceived rise in blood. The Barostim System is implanted in a safe and straightforward surgical procedure.
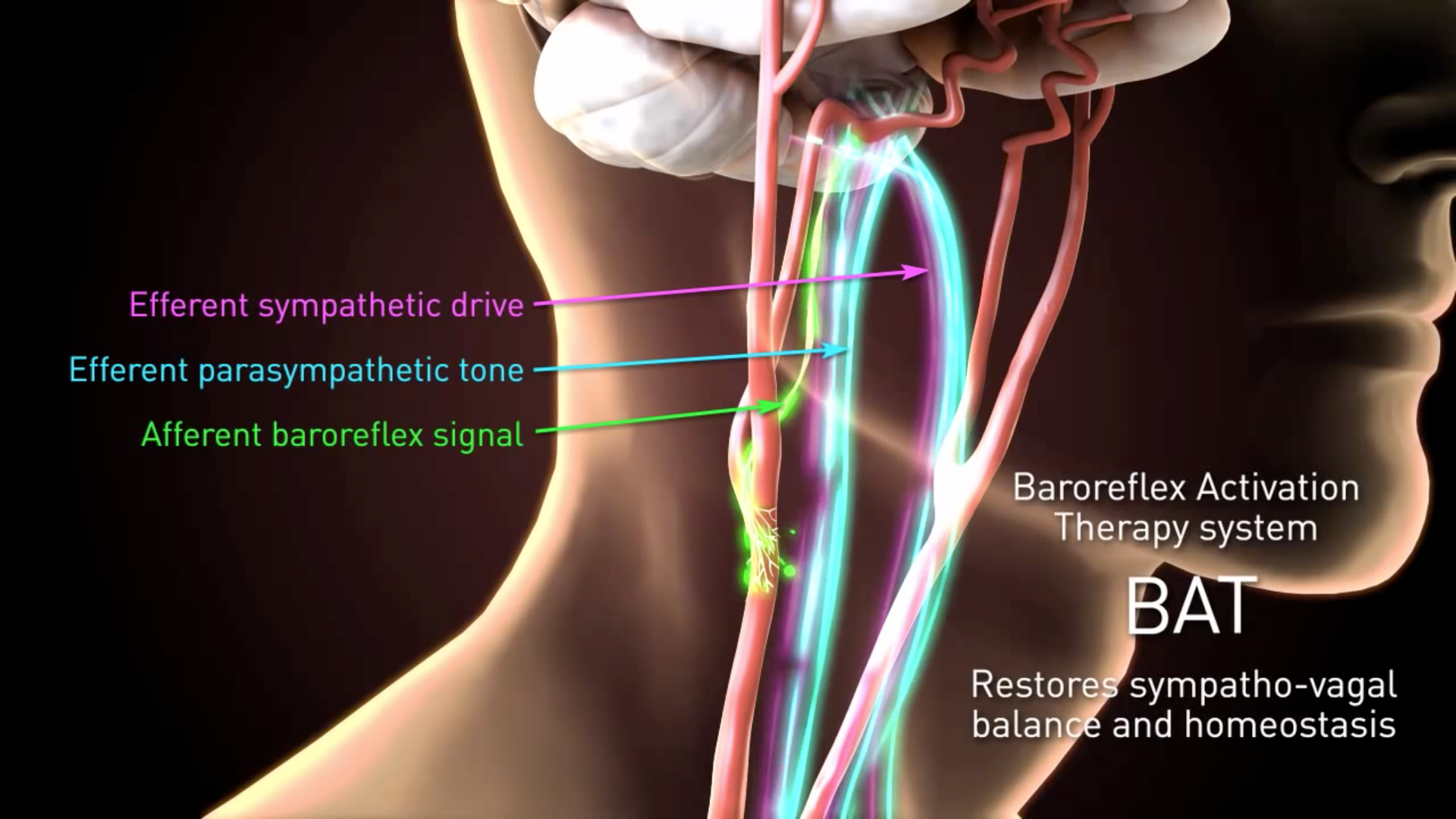
Barostim neo has on blood pressure and other hemodynamic parameters. The Barostim System Baroreflex Activation Therapy is delivered by the BarostimNEO an implantable pulse generator IPG designed to deliver continuous electrical stimulation to carotid baroreceptors. The device uses electrical impulses to stimulate the nerve that regulates blood pressure inducing the blood vessels to relax.
The brain then sends the necessary signals to the blood vessels and heart to reduce heart failure symptoms. The minimally-invasive neosystem uses CVRx patented Barostim Therapy technology to trigger the bodys own natural systems by electrically activating the carotid baroreceptors the bodys natural cardiovascular regulation sensors. All patients in the BeAT-HF phase 3 clinical trial were subjected to guideline-directed medical therapy including medication while 125.
The BAROSTIM NEO System Premarket Approval P180050 is a Class III carotid sinus stimulator an implantable medical device that delivers electrical signals to the bodys pressure sensors to. Barostim Neo - Baroreflex Activation Therapy for Heart Failure CMS Back to Approved IDE Studies Barostim Neo - Baroreflex Activation Therapy for Heart Failure Study Title Barostim Neo - Baroreflex Activation Therapy for Heart Failure Sponsor Name CVRx NCT Number NCT02627196 IDE Number G120010 CMS Approval Date 2016-06-23 Additional. After a simple mapping procedure the Carotid Sinus Lead is sutured to the carotid sinus.
Food and Drug Administration in 2019 following successful trials that were led by MUSC Health cardiologist Michael Zile MD. Food and Drug Administration FDA-approved device that uses a novel mechanism to improve heart function. The implanted Barostim Neo System sends electrical impulses to cells in the neck called baroreceptors.
Project Type Neuromodulation system Developer CVRx. Barostim Baroreflex Activation Therapy BAT uses the power of the autonomic nervous system to improve symptoms of heart failure. Barostim is a novel Congestive Heart Failure CHF treatment that uses the power of the brain to improve symptoms like breathlessness fatigue and swelling.
Seventy two subjects were randomized. When the baroreceptors are activated signals are se nt through neural pathways to the brain and interpreted as arise in blood p sure. 32 to the medical management arm and 40 to the device arm 38 implanted 2 withdrawn.
The system does not rely on patients adherence to recommended treatment regimen. As a result information is sent to the brain. The BarostimNEO generator is inserted in a standard device pocket and these procedures typically take less than an hour usually performed on an outpatient basis.
Anne Kroman Barostim won breakthrough device approval from the US. The system received CE mark from the National Standards Authority of Ireland NSAI in September 2014 to treat heart failure patients with an ejection fraction less than or equal to 35. The BAROSTIM NEO System is indicated for subjects with heart failure defined as New York Heart Association NYHA functional Class III and left ventricular ejection fraction LVEF 35 despite being treated with the appropriate heart failure guideline directed therapy.
Barostim improves quality of life 3X more than medications alone. A prospective randomized study describing the safety and efficacy of the BAROSTIM NEO System in heart failure subjects with left ventricular ejection fraction equal to or less than 35 percent. The Barostim System comprises the Barostim NEO IPG the Carotid Sinus Lead and a simple intuitive Programmer.
Barostim was estimated to be cost-effective compared with optimal medical treatment with an incremental cost-effectiveness ratio of 7 797QALY. Watch the video below to. Heart Blood Ti vessels Kidneys responder reduction.
BAROSTIM NEO is a US. Parameters assessed during visits are. The BAROSTIM NEO System is designed to electrically activate the carotid baroreceptors the bodys natural cardiovascular regulation sensors.
The neosystem is the CVRx next generation system for improving cardiovascular function. 100 Treatment Compliance Barostim neo provides 100 compliance to treatment by automatically and continuously activating the baroreflex. All subjects are now in long term follow-up and are required to have at least one annual visit.

Barostim Neo Neuromodulation Implantable System Usa

Fda Approves Device To Treat Patients With Heart Failure Wjar

A Barostim Neo Electrode Assembly And Implantable Pulse Generator Download Scientific Diagram

Innovative Device Therapie Der Herzinsuffizienz Management Krankenhaus

Cvrx Barostim Neo Now Cleared For Mri Use In Europe Medaxs

Investigational Device For Heart Failure Patients Stimulates Cells In Arteries To Improve Function Youtube

Barostim Neo Neuromodulation Implantable System Usa

Baroreflex Activation Therapy Barostim Neo System Tctmd Com

Ce Mark Approval For Heart Failure Treatment Today S Medical Developments

A Barostim Neo Electrode Assembly And Implantable Pulse Generator Download Scientific Diagram

Baroreflex Activation Therapy By The Rheos System And The Barostim Download Scientific Diagram

Barostim Baroreflex Activation Therapy Cvrx

Barostim Neo Neuromodulation Implantable System Usa
Barostim Neo 1 Radcliffe Cardiology

Baroreflex Activation Therapy Study In China 2022 Wiki English
First Implant Made For Barostim Neo Device To Treat Hypertension Daic

First Rheos And Second Barostim Neo Generation Baroreflex Download Scientific Diagram


Comments
Post a Comment